Sep
11
11
Drug- and lipid-resistant polymers are playing an increasingly important role in enhancing patient safety. Stringent sterilization techniques can cause cracking, crazing, and hazing in commonly used plastics. They can also have a yellowing effect on certain polymers, which can impact color-coding systems in connector applications.
Eastman Tritan™ copolyester is resistant to a wide array of medical fluids, such as oncology drugs, drug carrier solvents, and lipids. Along with its toughness, low residual stress, and color stability post-sterilization, Tritan is an excellent choice for fluid management components.
Regulations in the medical market are constantly changing. When Elcam Medical, a world-class manufacturer of disposable medical devices for the OEM market, wanted to further improve the safety and efficacy of its fluid management devices, they turned to Eastman to find a polymer that complies with new regulations while still optimizing performance.
Working with our technical experts, Elcam developed drug- and lipid-resistant BPA-free stopcocks and connectors made with Tritan. They are designed to meet new ISO 80369 standards, which encourage worldwide consistency in small-bore connectors for liquids and gasses in healthcare applications to reduce the risk of misconnections between medical devices and accessories.


Eastman Tritan™ copolyester is resistant to a wide array of medical fluids, such as oncology drugs, drug carrier solvents, and lipids. Along with its toughness, low residual stress, and color stability post-sterilization, Tritan is an excellent choice for fluid management components.
Regulations in the medical market are constantly changing. When Elcam Medical, a world-class manufacturer of disposable medical devices for the OEM market, wanted to further improve the safety and efficacy of its fluid management devices, they turned to Eastman to find a polymer that complies with new regulations while still optimizing performance.
Working with our technical experts, Elcam developed drug- and lipid-resistant BPA-free stopcocks and connectors made with Tritan. They are designed to meet new ISO 80369 standards, which encourage worldwide consistency in small-bore connectors for liquids and gasses in healthcare applications to reduce the risk of misconnections between medical devices and accessories.
For more information about Elcam Medical and their fluid management components made with Tritan, visit www.elcam-medical.com.

Blog categories:
Sep
4
4

Join us for Tritan on Tour—an exclusive event showcasing how new materials and manufacturing processes can improve medical device performance.
Hear new information about simulating, molding, bonding, and welding Eastman
Tritan™ copolyester.
Gain key insights on the latest with Tritan material and case studies; learn about speed to market through design for manufacturing/design for reliability (DFM/DFR).
Observe a Tritan tool running at the press and on-site presentations/demos from Nexeo Solutions, Beaumont Technologies, Henkel and Dukane!
Plus, we’ll begin the day with donuts and end with cocktails! Hope you can join in the fun!
Tritan on Tour—September 11
Advanced Molding Technologies
8700 Rendova Street NE
Circle Pines, MN 55014
View the full agenda and RSVP!
Advanced Molding Technologies
8700 Rendova Street NE
Circle Pines, MN 55014
View the full agenda and RSVP!
Blog categories:
Aug
15
15
 Small-bore connectors are important components of many enteral feeding devices. Good design is critical, as tubing misconnections or failure can put patients at risk for serious injury or death.
Small-bore connectors are important components of many enteral feeding devices. Good design is critical, as tubing misconnections or failure can put patients at risk for serious injury or death.Global design standards for tubing connectors are now helping improve patient safety and device efficacy. ISO 80369 requires small-bore connectors to be made of semirigid and rigid materials, making incorrect interconnections less likely. Enteral devices were the first of all the clinical applications to undergo this change.
To meet this standard, you may have to adjust your design, which means you may need a new mold or new materials. Eastman Tritan™ copolyester is a rigid material with the properties needed to comply with these regulations.
BPA-free Tritan demonstrates excellent chemical resistance, toughness, and color stability post-sterilization. It also helps maintain device safety and efficacy by ensuring that connecting parts retain their shape, dimension, and clarity during and after sterilization.
Using a rigid material that won’t be compromised by sterilization helps ensure patient safety and the efficacy of your device. Contact us for more information on switching to Tritan.

Blog categories:
Aug
9
9

Save the date!
Eastman and Advanced Molding Technologies are partnering to share innovative solutions to some of the biggest challenges in today's medical market.
Get ready for an information-packed day, including insights from leading experts: Advanced Molding Technologies, Eastman, Nexeo Solutions, Beaumont Technologies, Henkel, and Dukane.
Tritan on Tour—September 11,
Advanced Molding Technologies
8700 Rendova Street NE
Circle Pines, MN 55014
Blog categories:
Jul
20
20
As part of the continued effort to improve cancer treatment, pharmaceutical companies are developing new and improved oncology drugs. However, advanced oncology drugs and 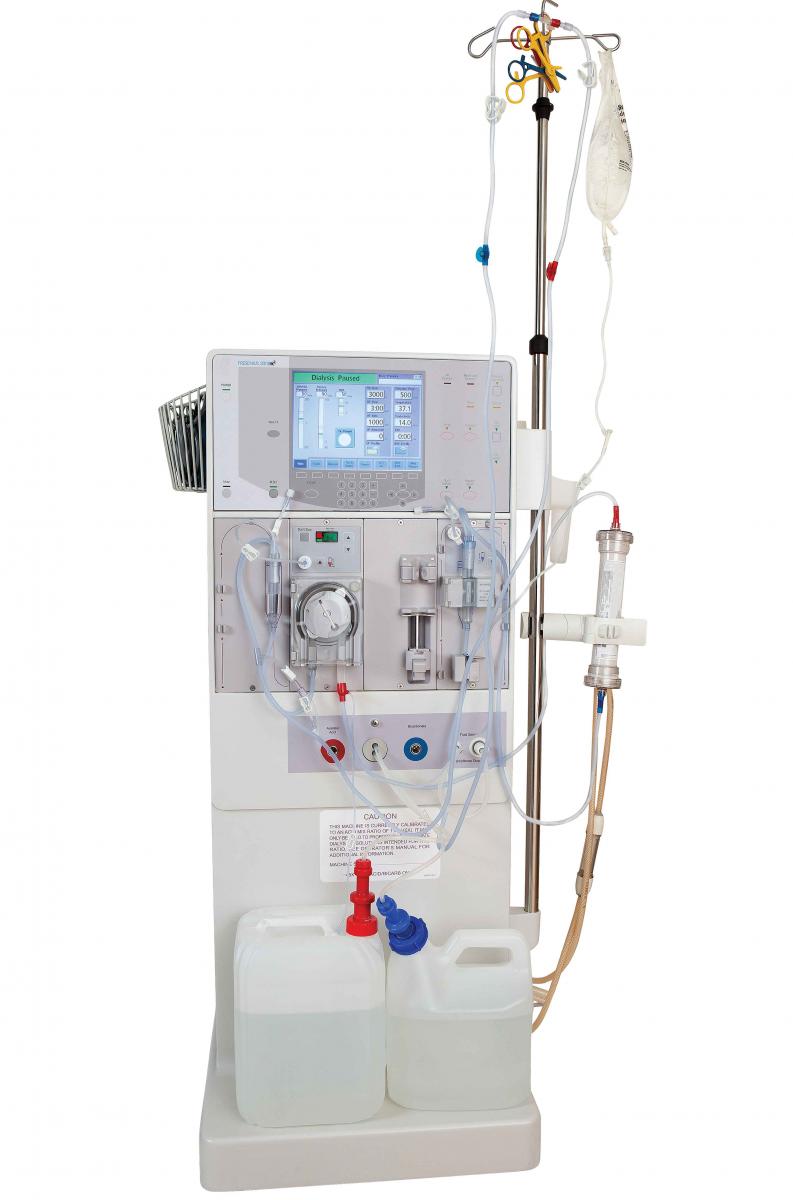 carrier solvents challenge the chemical resistance of polymers used in delivery devices. Such conditions can prevent devices from working properly or cause them to fail prematurely. When there is a pattern of compromised device performance or life cycle, regulatory agencies may tell manufacturers to stop using certain materials to protect patient safety.
carrier solvents challenge the chemical resistance of polymers used in delivery devices. Such conditions can prevent devices from working properly or cause them to fail prematurely. When there is a pattern of compromised device performance or life cycle, regulatory agencies may tell manufacturers to stop using certain materials to protect patient safety.
 carrier solvents challenge the chemical resistance of polymers used in delivery devices. Such conditions can prevent devices from working properly or cause them to fail prematurely. When there is a pattern of compromised device performance or life cycle, regulatory agencies may tell manufacturers to stop using certain materials to protect patient safety.
carrier solvents challenge the chemical resistance of polymers used in delivery devices. Such conditions can prevent devices from working properly or cause them to fail prematurely. When there is a pattern of compromised device performance or life cycle, regulatory agencies may tell manufacturers to stop using certain materials to protect patient safety.Polymer selection is critical to a medical device. Engineering polymers offer many advantages for infusion and blood contact devices compared with other materials. Advantages include design and color flexibility, aesthetic appeal, reduced weight, corrosion resistance, and clarity. But polymers that have a low level of compatibility with chemicals—such as lipids, disinfectants, and specific oncology drugs and solvents—can experience environmental stress cracking or premature device failure in the presence of applied or residual stress.
It’s important to evaluate polymers for chemical resistance to keep patients safe and ensure device longevity. Eastman Tritan™ copolyesters have good overall chemical resistance and provide an attractive alternative to polycarbonate (PC) or acrylonitrile-butadiene-styrene (ABS) for oncology drug delivery devices. For closed-system transfer devices and other infusion devices, Tritan can be a candidate for molding devices that are compliant with safety alerts from regulatory agencies such as the Food and Drug Administration (FDA) and the Institute for Safe Medication Practices.
Eastman technical specialists can help you early on in your process to produce high quality medical devices. Contact us to learn more about the attributes of Tritan and how it compares to other commonly used materials when tested for chemical compatibility.

Blog categories:




 Close
Close


