3
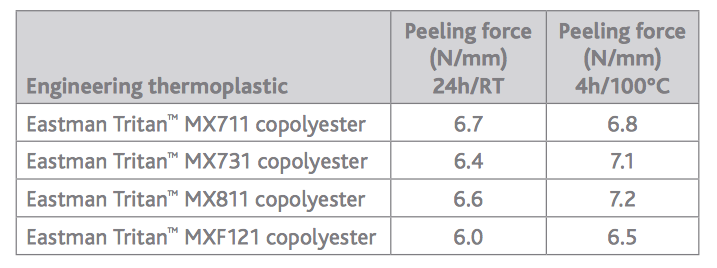
 We often receive requests from medical device developers and original equipment manufacturers for guidance on the best adhesives to use with Eastman TritanTM copolyester. To help customers achieve the best adhesive solutions, we partnered with Henkel Corporation to test various resins and adhesives for use in medical devices.
We often receive requests from medical device developers and original equipment manufacturers for guidance on the best adhesives to use with Eastman TritanTM copolyester. To help customers achieve the best adhesive solutions, we partnered with Henkel Corporation to test various resins and adhesives for use in medical devices.Henkel’s LOCTITE® adhesive continues to be tested at the industry’s most comprehensive ISO 10993 biocompatibility standards. Eastman looked to determine which resins and adhesives, when used with Tritan, could optimize a manufacturer’s assembly process. Results showed that the use of Tritan and LOCTITE together created superior results, including improved
curing to increased flexibility.
By using Eastman and Henkel products in conjunction, producers can combat safety issues such as breaking and cracking, resulting in fewer defects and tougher, longer-lasting products. Understanding the best adhesive option from the outset can also help clients eliminate the need for trials and testing, reducing production costs, and ultimately improve their bottom line.
For more about this partnership, check out our Building better bonds brochure.

1
Clear and opaque grades of Tritan have a lower Tg and require a lower processing temperature than other engineering polymers. Because Silopren LSR 47×9 can cure rapidly at relatively low temperatures, it’s possible to achieve optimal functional performance and efficient processing with Tritan.
This combination is ideal for applications that require properties like handling comfort, waterproofing, durability, and aging stability. Incorporating LSR technology enhances the advantages of Tritan, which include:
- Outstanding chemical resistance
- Excellent impact strength and durability
- Made without bisphenol A (BPA) and halogens
- Superior noise-damping characteristics
- Excellent clarity and color retention after sterilization
- Color match (with certain opaque grades)
- Design flexibility
4
When choosing a polymer for a medical device, it’s crucial to understand how the material will perform in the real world. Eastman’s 4-step test helps show how plastics hold up when exposed to frequent disinfection, but it’s also important to see how that translates into actual performance in the field.
That’s why we developed the housing drop test. This test can be used alongside the 4-step method to understand how a well-designed device will respond to impact after being disinfected.
Using materials commonly found in electronic medical device housings and hardware, we designed medical device housings with uniform wall thickness, gradual transitions, and smooth corners to help minimize stress. Each molded part was assembled with six screws using a fixed torque.
The devices were then submerged in Virex® TB for approximately two hours. To replicate use in the hospital environment, we dropped each device multiple times from a height of three feet and visually inspected for cracks and breakage.
The results of this test match closely with results from the 4-step test. To see the test in action and learn more about how different material performed, check out this video.
14
This simple, easily-repeatable test can help predict the reliability of a material after exposure to harsh cleaners and drugs commonly used in hospital settings. The method uses a 1.5% constant strain jig together with wet patches for applying chemical reagents. Here’s how it works:
- Select the appropriate jig.
- Load flex bars onto jig.
- Apply chemicals to the flex bars.
- Perform reverse side impact test.
Read our Disinfect with Confidence brochure for more details on each step of the process, and check out this video.
Ultimately, this test should help you confidently choose the best material for your next project.
Learn more about Eastman’s medical grade polymers for medical device housings and hardware at Eastman.com/medicalhousings.

6
Tritan on Tour took a trip across states to UBM Advanced Manufacturing Expo in Anaheim, CA where Eastman's next-generation polymer for the medical market was showcased to an intrigued crowd. Learn more about the new polymer by visiting http://www.plasticsnews.com/article/20180223/NEWS/180229943/eastman-emphasizes-value-of-disinfectant-ready-polymers.




 Close
Close