Dec
10
10

Eastman is excited to exhibit at Medical Design & Manufacturing (MD&M) West 2020! Join us February 11–13 in Anaheim, California, as we share exciting new innovations that are helping improve medical equipment and create positive health outcomes.
At MD&M West 2020, we’ll be highlighting the latest advancements in medical technology and the collaborations that are key to success in the medical market. Don’t miss out any of the fun:
- Stop by booth 2301 to learn about four new products as part of the Eastman Tritan™ MXF portfolio of medical polymers.
- Attend our Lunch and Learn events to discover what industry experts are saying about the future of medical devices and packaging.
- Register for one-on-one sessions with members of Eastman’s medical device and packaging teams.
MD&M West 2020
Feb. 11–13, 2020
Anaheim Convention Center
Anaheim, California
Booth 2301
Feb. 11–13, 2020
Anaheim Convention Center
Anaheim, California
Booth 2301
Blog categories:
Nov
22
22

As healthcare facilities ramp up the use of aggressive disinfectants, traditional plastics are feeling the strain. It’s all too common to see device housings become sticky; wear thin in high-touch areas; or crack, crumble, or shatter after only a few months of service. The issue? Devices that were designed just a few years ago are typically made with materials that lack the right combination of impact strength and chemical compatibility with today’s stringent cleaning protocols.
Eastman Tritan™ copolyester is an excellent choice for medical housings due to its superior toughness and high chemical resistance. Using Tritan in your device can also help prevent costly repairs and replacements, keeping patients safe and customers happy.
In our webinar “Many Medical Hardware Housing Complaints. One New Technology Solution,” we share an in-depth look at how device performance is being compromised by the increased use of aggressive disinfectants and how material choice can make a key difference in device success.
Watch it on demand to learn more about:
- The true cause of part failures and how to address it
- Strategies to improve device reliability, reduce repair costs, and extend product life
- Test results comparing Tritan’s performance with that of traditional housing materials
Blog categories:
Oct
21
21

You want to create medical devices and packaging that ensure patient safety and provide long-lasting reliability. To accomplish that, you have to choose high performance medical grade materials. Manufacturers who specify medical grades of Eastman Tritan™ copolyester not only get access to advanced, high-quality raw materials but also to a strong level of support throughout the regulatory journey to commercialization of a new product.
By specifying Eastman medical grade polymers, you get help with:
Eastman has long history as reliable global supplier of raw materials. We provide not only leadership in innovation and business continuity but also regulatory support and processes that help customers take products to market with greater efficiency and confidence. For more information on the support services we provide, contact an Eastman customer service representative.
By specifying Eastman medical grade polymers, you get help with:
- Biocompatibility
- Sterilization
- Quality systems
- Dedicated regulatory support
- Regulatory statements and product regulatory information sheets
- FDA Drug Master Files
- Quality systems and cGMP
- Application development and technical services
- Educational webinars and customized lunch and learn sessions
- Design recommendations
- Material FFU criteria and specific performance testing
- Competitive materials analysis or ID
- Physical property testing
- Aging and sterilization studies
Eastman has long history as reliable global supplier of raw materials. We provide not only leadership in innovation and business continuity but also regulatory support and processes that help customers take products to market with greater efficiency and confidence. For more information on the support services we provide, contact an Eastman customer service representative.
Blog categories:
Sep
23
23
Traditionally, the world operates in a linear economy—raw materials are used to make products, which are then used and disposed of as waste. A circular economy focuses on making the most of the world’s resources by providing end-of-life solutions to reduce, reuse, and recycle products and materials that typically end up in landfills and waterways.
For manufacturers, there’s a lot to consider when making the shift to circular processes or materials. Eastman has developed a unique platform of solutions focused on minimizing waste and maximizing value to support the evolution of the circular economy. Our portfolio of circular solutions includes:
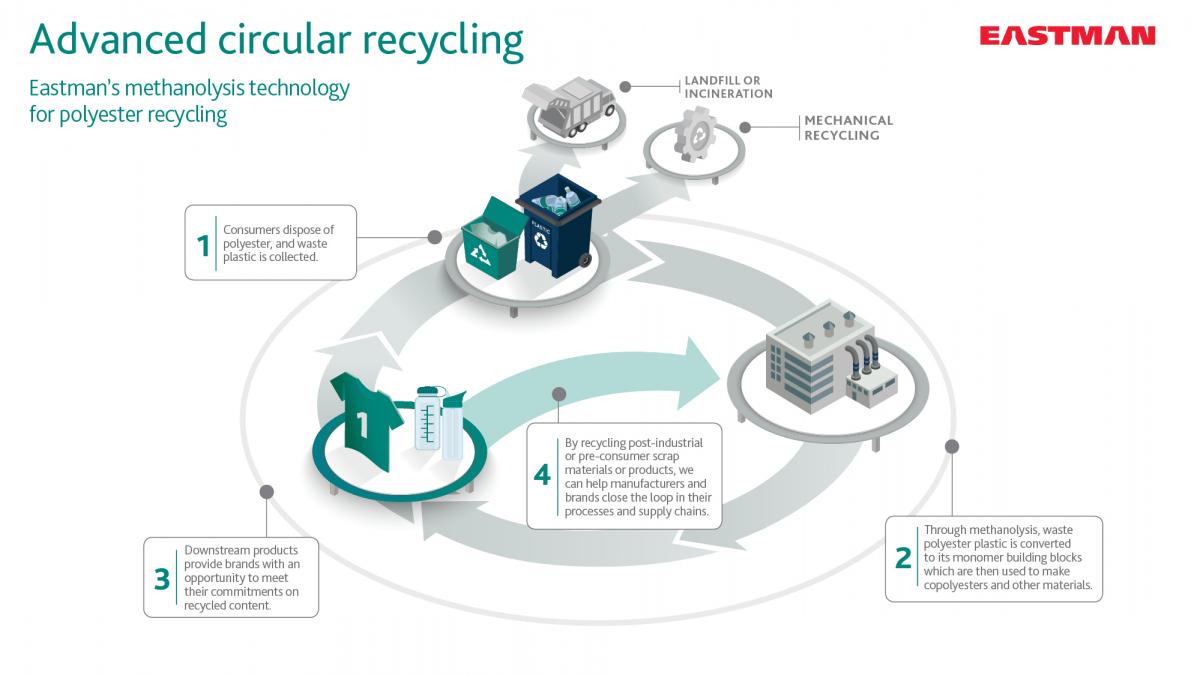
Advanced circular recycling (ACR)
This operation involves chemical recycling on a broadly mixed stream of polyester-family polymers using a process called methanolysis. Through methanolysis, polyester materials are taken back to their polymer building blocks. These building blocks can then be reintroduced to the production of new polyester-based polymers, delivering a true circular solution. ACR takes low-quality polyester waste that would typically be diverted to landfills and instead recycles it into high-quality polyesters suitable for use in a variety of end markets.
For manufacturers, there’s a lot to consider when making the shift to circular processes or materials. Eastman has developed a unique platform of solutions focused on minimizing waste and maximizing value to support the evolution of the circular economy. Our portfolio of circular solutions includes:
Advanced circular recycling (ACR)
This operation involves chemical recycling on a broadly mixed stream of polyester-family polymers using a process called methanolysis. Through methanolysis, polyester materials are taken back to their polymer building blocks. These building blocks can then be reintroduced to the production of new polyester-based polymers, delivering a true circular solution. ACR takes low-quality polyester waste that would typically be diverted to landfills and instead recycles it into high-quality polyesters suitable for use in a variety of end markets.
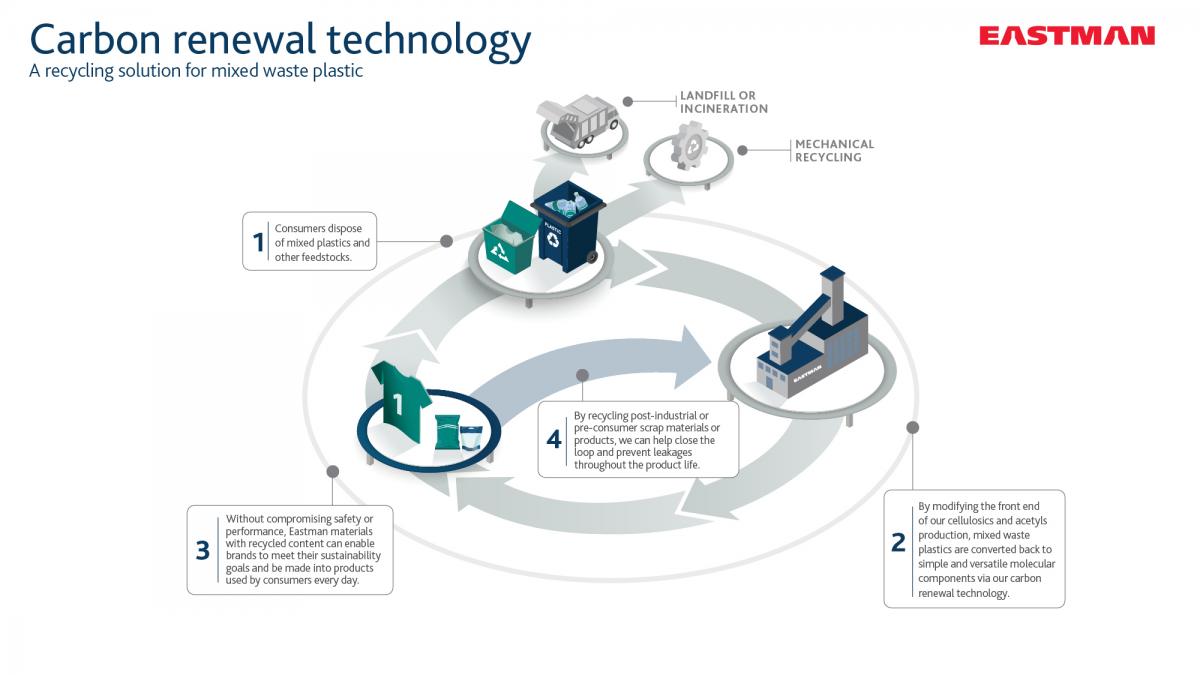
Carbon renewal technology (CRT)
This technology helps divert materials such as flexible packaging and plastic films away from landfills. By modifying the front end of Eastman’s cellulosics production stream, CRT converts plastic waste back to simple and versatile molecular components. The process partially oxidizes the waste plastic, converting the feedstock input at very high efficiency back into the basic building blocks of Eastman’s cellulosics product lines that serves an array of industries.
This technology helps divert materials such as flexible packaging and plastic films away from landfills. By modifying the front end of Eastman’s cellulosics production stream, CRT converts plastic waste back to simple and versatile molecular components. The process partially oxidizes the waste plastic, converting the feedstock input at very high efficiency back into the basic building blocks of Eastman’s cellulosics product lines that serves an array of industries.
As the world transforms, these new technologies will help unlock value in waste plastics, which currently cannot be recycled or are difficult to recycle. For more information on Eastman’s role in the circular economy, visit our website!
Blog categories:
Aug
23
23

With this handy app, users can:
- Calculate drying time
- Calculate melt residence time
- Calculate shot capacity utilization
- Download data sheets
- Watch videos
- Get advice from the Tritan experts
Download the free Tritan Mold It app from the App Store today.

Blog categories:




 Close
Close






