14

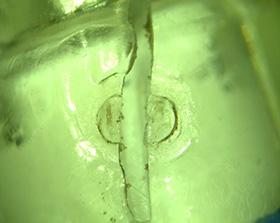
Gates are the belly buttons of the injection molding world—every molded part has one. The big difference is that molders can control the location of the gate. The location should be selected based on an evaluation of part aesthetic requirements, mechanical loading requirements, and fill pattern.
Locating the gate for aesthetics and mechanical properties
Aesthetics
- The gate location on an injection molded part leaves a “witness” where the part is separated from the runner system.
- This is considered an appearance defect and is typically hidden in an inconspicuous location on the part.
Mechanical properties
- Resin enters the molding cavity at high pressure and high temperatures at the gate location.
- The part surface in the gate area typically includes defects that can behave as stress concentrations during tensile loading or drop testing.
- Gate locations typically exhibit inferior mechanical properties compared to the molded resin in the rest of the cavity.
- Gate locations should be located in areas of the part which are not subjected to externally applied high-tensile loading.
| TMI TIP: Learn more about gate location and other ways to improve manufacturability through part and mold design. Watch a recent webinar hosted by Eastman. |
2
Again this year, Eastman will be exhibiting at IHHS—International Home and Housewares Show—in Chicago, IL.
Join us at Booth S4875 from Mar. 5 to 8 to learn all the ways that Tritan™ from Eastman is clearly better for the housewares and durables markets than other materials.
And you’re invited to a swing at the Eastman Batting Cage—where you can test Tritan toughness and durability firsthand.

While you’re at the Eastman booth, you can:
- See how Tritan stacks up in performance testing against other plastics.
- See demonstrations of the safety and reliability of products made with Tritan.
- See which exhibitors at IHHS are now using Tritan from Eastman.
17
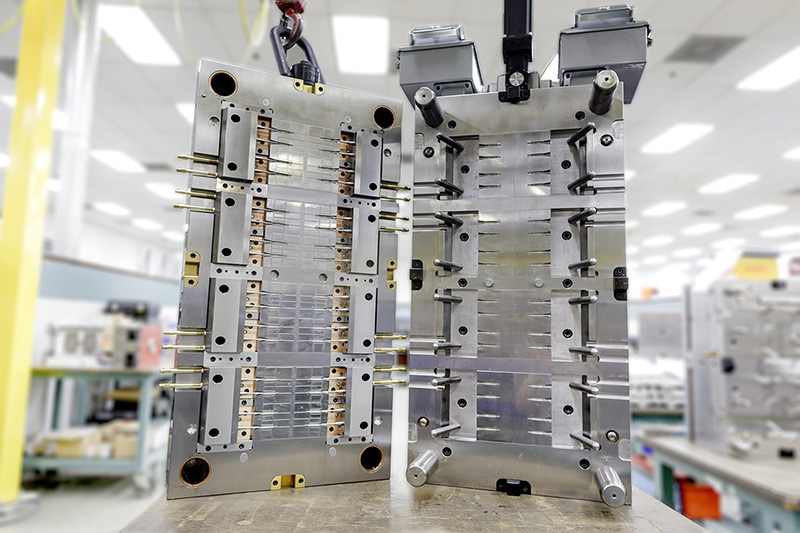
There’s a big incentive for molders of small medical parts to increase production efficiency by going to 16, 32, or even more cavities using hot runner/valve gate systems. Molders have always had great success with Eastman Tritan™ copolyester for medium-to-large components of medical devices and electronic instruments. Recently, our commitment to small, multicavity solutions began delivering big results.
Here are a few issues to consider when designing molds for multicavity production.
Residence time
- As cavitation is increased, the distance from the machine nozzle to the cavities increases. This results in a greater volume of resin in the hot runner manifold and, subsequently, longer residence times. For example, in a recent 32-cavity mold, the weight of the material in the hot runner system is 22 times the weight of all 32 parts combined.
- Eastman's guideline for design residence time with copolyesters is 5 minutes.
Pressure drop
- Higher pressure losses through the runner system are typical of higher-cavitation tooling, because the increased distance from the machine nozzle to the cavities results in longer flow-length requirements for the resin.
- Eastman's guideline for maximum fill pressure for a runner/part/gate is 20,000 psi.
Balance
- Higher-cavitation tooling can make it more difficult to achieve cavity-to-cavity balance during the filling process. This can affect part quality and increase scrap rate.
- Recently, we solved this problem with a synchro-plate system that mechanically actuates all valve gates simultaneously. Contact Eastman for more details.
Venting and cooling
- Inadequate cooling and venting can result in high levels of residual stress and increased warpage.
- Increasing cooling channel diameters without maintaining velocity can actually result in a decrease in the total heat removed in a given channel.
- When molding Tritan, it is critical to freeze the outer skin thoroughly before ejection to prevent sticking and deformation of the part. (See Jan. 5 blog on “Proper Cooling.”)
Mold finish
- Some engineering polymers are very sensitive to mold finish, making mold polishing critical.
- In some cases, surfaces polished smoother than required for ejection only add to the mold cost. What’s more, highly polished surfaces can create a vacuum in low-or no-draft areas and actually hinder ejection.
- Tritan is less sensitive to mold finish—and in some cases has run successfully with less thorough mold polishing
You can see more information about mold design under either the MEDICAL or DURABLES tab above.
|
TMI TIP: As always, early collaboration helps ensure best results from high-cavity molds. Watch this unique collaboration video. |
You can learn more about how Eastman is helping customers achieve optimal tooling, processing, and manufacturing results in the August 2015 issue of Plastics Technology.
1
You can speak with a Tritan expert in person at MD&M West.
Eastman will be exhibiting in Booth 2407, February 9 to 11. Stop by to see how Eastman Tritan™ copolyester and other medical-grade polymers are helping device makers meet regulatory requirements, improve patient safety, and ensure confidence in end-product performance.
While at our booth, you can pick up two white papers with great information about important roles chemical resistance plays in preventing health care-acquired infections and complying with best practices for oncology drug delivery devices.
MD&M West will be the center of the medtech world in early February. It’s a great place to discuss possibilities our whole portfolio of medical-grade polymers. You’re invited to talk with Tritan experts about specific advantages for medical devices, including:
• Toughness
• Crystal clear or opaque
• Chemical resistance
• Sterilization stability
• Made without bisphenol A (BPA)
• UL V2 flame rating
• Successful secondary operations
| TMI TIP: To schedule a private appointment during your booth visit, go to www.Eastman.com/MDMW2016. |
18
Some traditional polymers used in drug delivery devices are not compatible with modern oncology chemotherapies—including the cancer drugs and the carrier solvents that help make them effective.
Device manufacturers have more reasons than ever to understand the chemical resistance of the materials they use in devices, including:
• The widespread use and growing economic importance of oncology drugs
• A recent FDA Safety Alert* concerning infusion devices made with polycarbonate (PC) or acrylonitrile-butadiene-styrene (ABS)
All stakeholders are working together to reduce product failure and improve safety through:
• Vigilance by regulatory agencies
• Chemical resistance research by polymer manufacturers
• Informed polymer selection for oncology drug delivery devices
For its part, Eastman has conducted a series of chemical resistance tests on Eastman Tritan™ copolyester and competitive polymers. The test results are presented and discussed in a recently posted webcast titled “Why Devices are Failing in Oncology Drug Delivery Applications”.
Eastman also offers a free white paper that will give you more details of the testing.




 Close
Close


